Cannabinoid Insight has monitored the various numbers and statements made since the publication of the CBD list by the Food Standards Agency (FSA) in March. Here is how things stand in April 2022, in the weeks after the list went live.
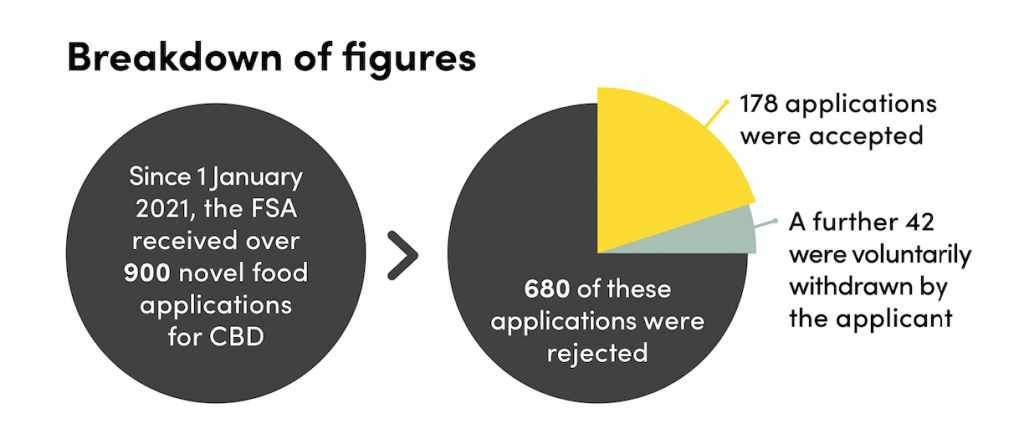
Since 1 January 2021, the FSA has received over 900 novel food applications for CBD on sale in England and Wales. Approximately 680 of these applications were rejected and a further 42 were voluntarily withdrawn by the applicant.
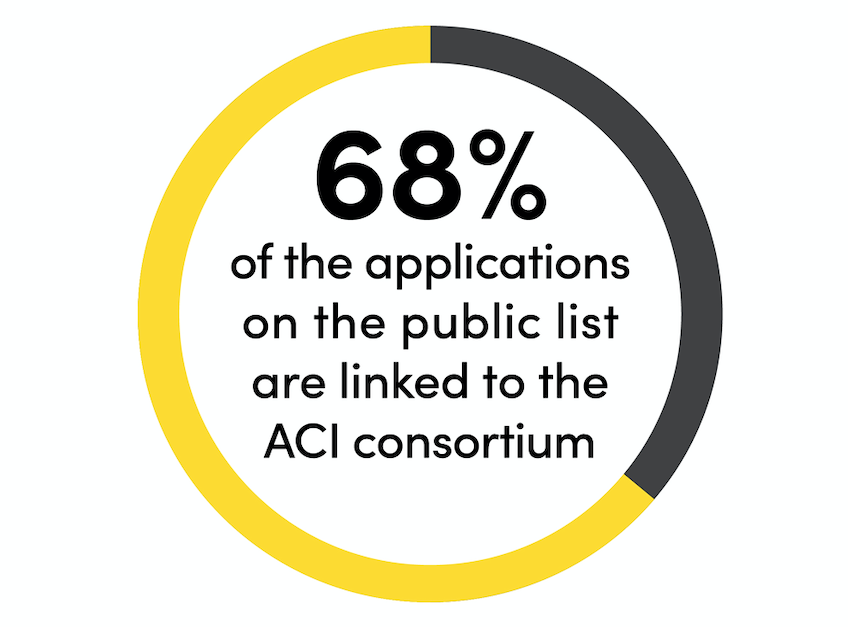
On 31 March 2022, the FSA released a list of permitted CBD products, which will remain available on sale, pending full market authorisation expected in 2023. This list consists of 3,536 ingestible CBD products, with 68% of these linked to applications submitted via the Association for the Cannabinoid Industry Novel Foods Consortium.
Just seven companies find themselves validated, as of April 2022. In the FSA’s regulated products system, the pathway which companies use to submit their novel foods applications, there are 182 live applications, including the 70 applications mentioned above. Of the 182 applications, 175 are understood to be in the pre-validation stage.
What happens next?
Those who have been validated, and any future validated applications, will progress towards market authorisation. For those in the pre-validation stage, the FSA said in a press conference that this process is expected to take up to nine months, with some delays expected. This means that it will be early-to-mid 2023 before the first fully authorised CBD product emerges in the UK.
In the meantime, all businesses operating with CBD products are being urged to check the products in their inventory. The FSA database can be found here. Retailers and consumers are being urged to use UKCBDList.com, a free resource created by the ACI.
Initial enforcement will be on the basis of voluntary withdrawal. Trading Standards is urging retailers and consumers to bring non-compliant products to their attention.





